What is COVID-19 IgG/IgM Rapid Test Kit?
COVID-19 IgG/IgM Rapid Test (Whole Blood/ Serum/Plasma) is a rapid chromatographic immunoassay for the qualitative of IgG and IgM antibodies to COVID-19 in human whole blood, serum or plasma as an aid in the diagnosis of primary and secondary COVID-19 infections.
- Rapid testing for SARS-CoV-2 antibodies within 10 minutes
- Just 10ul of specimen : Whole blood, serum , plasma
- Suitable for Point of Care Testing. No need for extra equipment
- For suspicious patients with symptoms, mild symptoms, or even without symptoms, also for testing people with close contact of infected patients and people under quarantine control.

What does the COVID-19 IgG/IgM Rapid Test detect?
The test detects the presence of patient-generated antibodies against SARS-CoV-2, the virus which causes the disease COVID-19. The test can detect two types of antibody isotypes: IgG and IgM.
IgM antibodies are the first antibodies to appear in response to a novel antigen. They imply a more recently initiated infection.
IgG antibodies have a higher affinity for the target antigen, meaning they are more specifically able to bind the substance which caused the immune response. IgG antibodies are generated later in the course of infection.
IgM and IgG antibodies can both be present in a sample. This implies that the conversion from a primarily IgM to IgG humoral response is underway.
A sample can be positive if there are IgM, IgG, or both IgM and IgG antibodies present.
How does the COVID-19 IgG/IgM Rapid Test work?
It is widely accepted that Immunoglobulin M (IgM) provides the first line of defence during viral infections, followed by the generation of adaptive, high affinity Immunoglobulin G (IgG) responses for long-term immunity and immunological memory. Therefore, testing of COVID-19 IgM and IgG antibodies is an effective method for the rapid diagnosis of COVID-19 infection. Detection of IgM antibodies tends to indicate a recent exposure to COVID-19. Detection of IgG antibodies indicates a later stage of infection. The testing device has a strip, which contains a colloidal gold-labelled recombinant novel coronavirus antigen and quality control antibody colloidal gold marker, two detection lines (G and M lines) and one quality control line (C) fixed on a nitrocellulose membrane. M is fixed with monoclonal anti-human IgM antibody for detecting the novel coronavirus IgM antibody. G is fixed with monoclonal antihuman IgG antibody for detecting the novel coronavirus IgG antibody.
How accurate is the COVID-19 IgG/IgM Rapid Test?
In order to test the detection sensitivity and specificity of the COVID-19 IgG-IgM combined antibody test, blood samples were collected from COVID-19 patients from multiple hospitals and Chinese CDC laboratories. The tests were done separately at each site. A total of 615 cases were tested: 403 (positive) clinically confirmed (including PCR test) COVID-19-infected patients and 212 non- COVID-19-infected patients (212 negative). The testing results of vein blood without viral inactivation were summarized in the Table 1. Of the 403 blood sample from COVID-19-infected patients, 397 tested positive, resulting in a sensitivity of 98.511%. 25 of the blood samples from the 212 non-COVID-19 infection patients tested positive, generating a specificity of 88.208%.
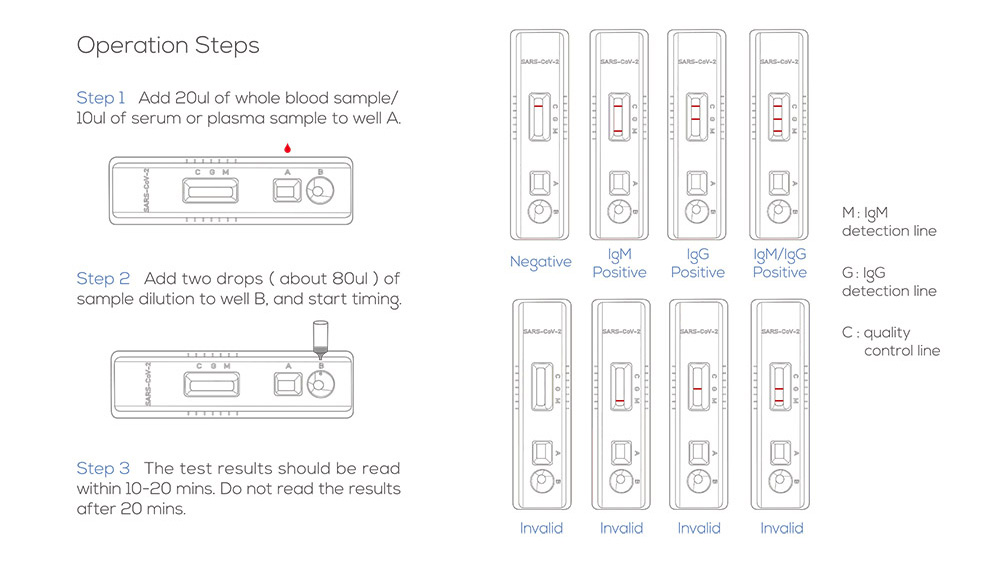
COVID-19 IgG/IgM Rapid Test Operation Steps
Steps:
- The whole blood, serum, or plasma clinical specimen is added to the well. Then, the dilution buffer (10 mM phosphate-buffered saline) is added.
- The combined sample flows down to the sample pad.
- Capillary action/lateral flow will move the sample across the test.
- The sample will hit the conjugation pad. The conjugation pad contains the COVID-19 antigen conjugated to 40 nm gold nanoparticle (AuNP) colloid. During this stage, any antibodies in the sample with specificity for COVID-19 will bind the antigen and its conjugated gold nanoparticle. (The kit control, rabbit IgG, is conjugated to the same gold nanoparticle and travels with the rest of the sample from this step.)
- Next, the sample/conjugate complex moves to the nitrocellulose membrane. Here, it comes in contact with the three test lines: IgG, IgM and control.
- First is the M line, which contains an immobilised antibody that recognises human IgM. Any IgM antibodies will bind here. However, only human IgM antibody/COVID-19 antigen/gold nanoparticle complexes will produce a visible coloured line.
- Second is the G Line, which contains an immobilised antibody that recognises Human IgG. All IgG antibodies will bind here. However, only human IgG antibody/COVID-19 antigen/gold nanoparticle complexes will produce a visible coloured line.
- The control line is the last line the sample will encounter. The control line contains an immobilised antibody that recognises Rabbit IgG, the control antibody. To serve as a procedural control, a coloured line should always appear in the control line region, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
- Finally, any excess will flow through to the absorption pad.
- After 10 minutes, the results of the test can be read.
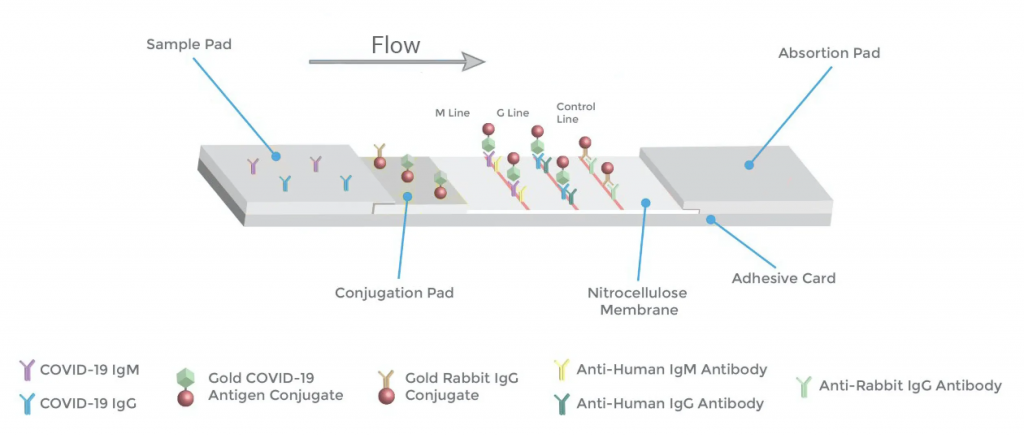
COVID-19 IgG/IgM Rapid Test Principle
This COVID-19 Rapid Point of Contact CE-IVD test consists of two components, an IgG and an IgM. In the IgG component, anti-human IgG coats the G test line region. In the IgM component, anti-human IgM coats the M test line region.
During testing, the specimen reacts with SARS-CoV-2 antigen-coated gold nanoparticles (AuNP) in the conjugation pad of the test cassette. Any antibody in the patient sample that recognises the MK201027 SARS-CoV-2 antigen binds to the Antigen-AuNP complex. The mixture then migrates laterally across the membrane by capillary action/lateral flow.
As these hAb-Antigen-AuNP (AbAgAu) complexes move across the test lines, they are captured by the anti-human IgM (M Line) or anti-human IgG (G Line) antibodies.
The sample first reaches the anti-human IgM antibodies which coat the M line. If the specimen contains IgM antibodies to SARS-CoV-2, a coloured line will appear in the M test line region.
Next, the sample reaches the anti-human IgG antibodies which coat the G line. If a specimen contains IgG antibodies to SARS-CoV-2, the conjugate-specimen complex reacts with anti-human IgG. A coloured line appears in the G test line region as a result.
Only human antibody/SARS-CoV-2 Antigen/AuNP complexes will produce a visible red/pink line at the M or G Line. Other antibodies produce no colour.
The rabbit IgG-AuNP complexes are captured by the control line (which contains anti-rabbit-IgG). This visible line indicates that there has been successful lateral flow across the detection strip. It is last to ensure that the sample had sufficient volume to move across the entirety of the test cassette.
Excess Antigen-AuNP complex will not be captured by the M or G lines. If no anti-MK201027 antibodies present in patient sample, no Ag-AuNP complex will be captured at the M or G Lines, and so no coloured line appears
To summarise, if the specimen contains SARS-CoV-2 IgG antibodies, a coloured line will appear in IgG test line region. If the specimen contains SARS-CoV-2 IgM antibodies, a coloured line will appear in IgM test line region. If the specimen does not contain SARS-CoV-2 antibodies, no coloured line will appear in either of the test line regions, indicating a negative result. In all cases, a coloured line should appear at the control, C line.
What influences the COVID-19 IgG/IgM Rapid Test testing results?
Please make sure the kit is recovered into room temperature and perform the kit under the room temperature(15℃~30℃). The results will be affected by high or low temperature. More About COVID-19 IgM/IgG Rapid Test Kit

